Polpharma utilizes up-to-date technologies, which ensure the safety of products,
employees, and the natural environment.
We have the capabilities to provide customers with materials ranging from clinical quantities up to in-market supply. We have full and vertical integration of processes, so that cost-effective and on-time solutions are delivered to our partners. API manufacturing is carried out in cGMP-compliant and FDA-approved multipurpose plant.
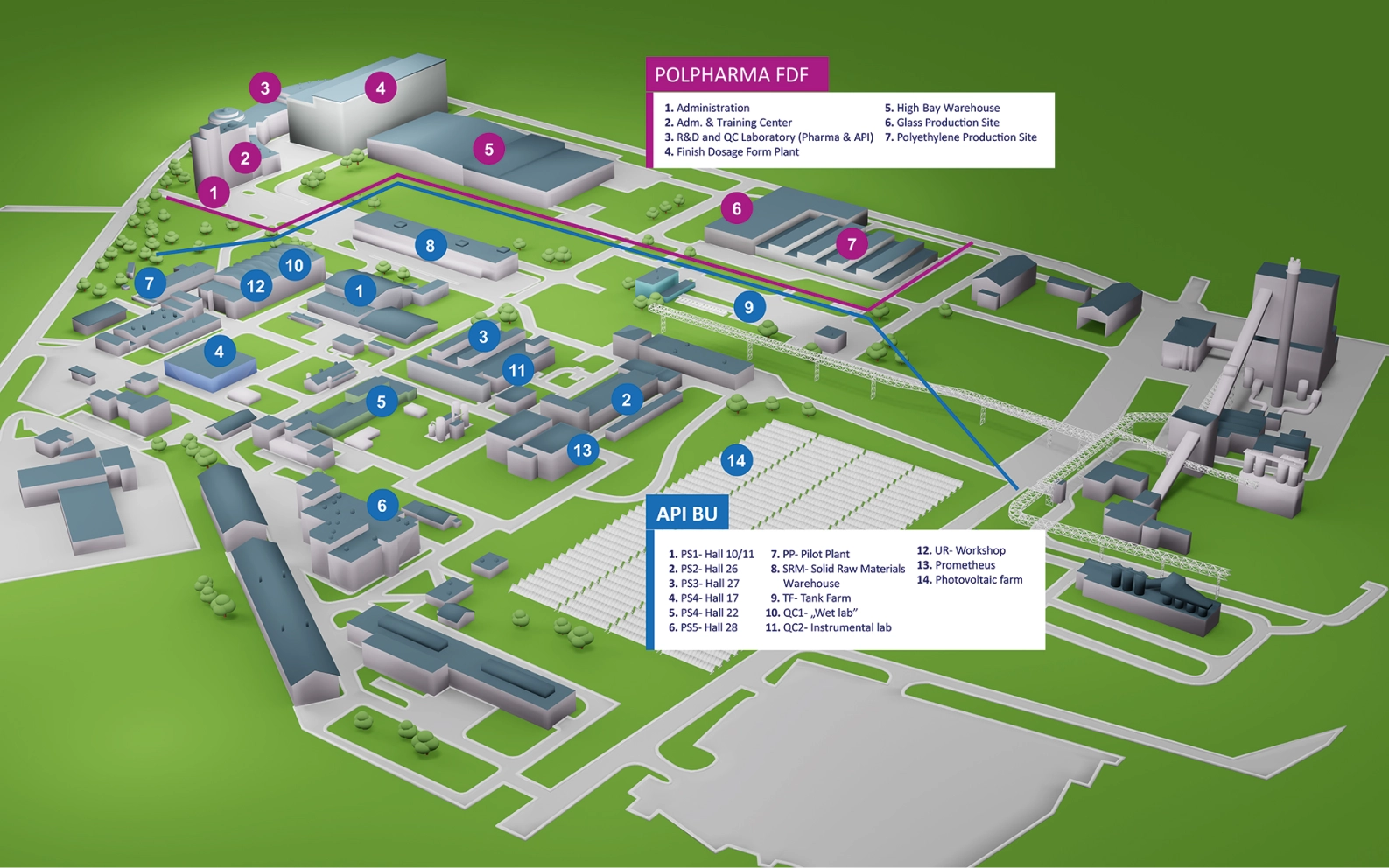
Starogard Gdański Site View


33,1 ha
Manufacturing Site
6,2 ha
Area dedicated to Polpharma API production
4,1 ha
Wastewater treatment plant
3 ha
Warehousing (tank farm and solid raw materials)
123.498 m2
Total Building Surface
19.246 m2
Polpharma API production
10.735 m2
Warehousing
6
Multipurpose buildings,
Pilot Plant, R&D
Laboratories
Manufacturing plant
Our manufacturing plant located in Starogard Gdanski – 47 km from the international airport in Gdansk – is an FDA approved multipurpose facility for API and intermediates production with a total capacity exceeding 350 m3.
Polpharma is the largest Polish manufacturer of active pharmaceutical ingredients. Our substances are available in over 60 countries on six continents.
With reactor volume from 150 L to 6 000 L in 6 interconnected buildings, operating from -50°C to +200°C pressure up to 10 bar, and highly automated units for reaction parameters monitoring and control, we are being positioned among top European API CMOs. Polpharma can accommodate a broad range of chemical processes to support customers in contract development and contract manufacturing projects.
Segregated clean areas with a different types of equipment for isolation (nutch filters, centrifuges) and drying (filter driers, tray driers, vacuum driers). Milling and micronization to adapt particle size distribution to required customer needs.












